+++ Achtung: diese Nachricht ist nicht mehr gültig +++
Nach der Rücknahme des Zulassungsantrages für Edaravone in der EU durch den pharmazeutischen Hersteller Mitsubishi Tanabe Pharma wurde das Evaravone-Programm beendet.
Im Mai 2017 wurde das Medikament Edaravone zur Behandlung der ALS in den USA zugelassen (Handelsname: Radicava™). In Deutschland ist Edaravone – bis zur möglichen Zulassung – durch Einzelimport über internationale Apotheken gemäß § 73 Arzneimittelgesetz grundsätzlich verfügbar. Bisher steht Edaravone ausschließlich in einer intravenösen Darreichungsform zur Verfügung.
Die Behandlung mit Edaravone erfolgt in Behandlungszyklen. Ein Behandlungszyklus umfasst 28 Tage. Der Zyklus besteht aus 14 Tagen, in denen an 10 Tagen Infusionen absolviert werden müssen, sowie 14 Tage einer Behandlungspause.
Durch den Import und die kontinuierliche Fortführung der Infusionen ist die Edaravone-Behandlung mit entsprechenden zeitlichen und logistischen Aufwendungen verbunden. Das Edaravone-Management-Programm (EMP) unterstützt Patienten und Ärzte bei der komplexen Anwendung des Medikaments im ambulanten Bereich.
- Vernetzung und Informationsaustausch von Patient, Arzt und Apotheker über die Internetplattform Ambulanzpartner
- Unterstützung bei der Versorgung
- Monitoring des Krankheitsverlaufs und der Behandlungszufriedenheit
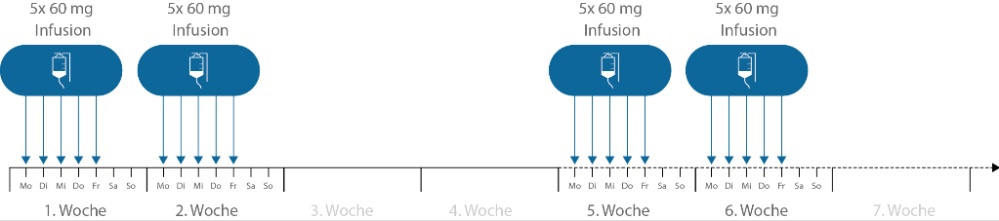
Abbildung 1: Zeitliche Abfolge der Edaravone-Behandlung
Vernetzung von Patient, Arzt und Apotheker
Nachdem die Entscheidung zur Behandlung mit Edaravone von einem Facharzt für Neurologie getroffen wurde, erfolgt die Antragstellung auf Kostenübernahme bei der Krankenkasse. Mit Bewilligung der Kostenübernahme muss der Import von Edaravone und die ambulante Infusionsbehandlung organisiert werden. Eine Abstimmung der beteiligten Personen ist daher von besonderer Bedeutung.
Das EMP vernetzt und unterstützt Ärzte, Apotheker sowie Patienten und Angehörige vor und während der Behandlung mit Edaravone. Mit dem EMP werden Daten zum Import, Logistik, Versorgung und Krankheitsverlauf über die Internetplattform Ambulanzpartner erhoben und allen Beteiligten bereitgestellt.
Unterstützung bei der Versorgung
Mit dem EMP erhalten Patienten, die bei ihrem Arztbesuch verordnete Edaravone-Therapie nach Hause geliefert, ohne selbst eine Apotheke aufsuchen zu müssen. Durch den fristgerechten Import des Arzneimittels kann eine laufende Edaravone-Therapie zuverlässig fortgeführt werden. Damit ermöglicht das EMP eine bundesweit einheitliche und effektive ambulante Edaravone-Versorgung.
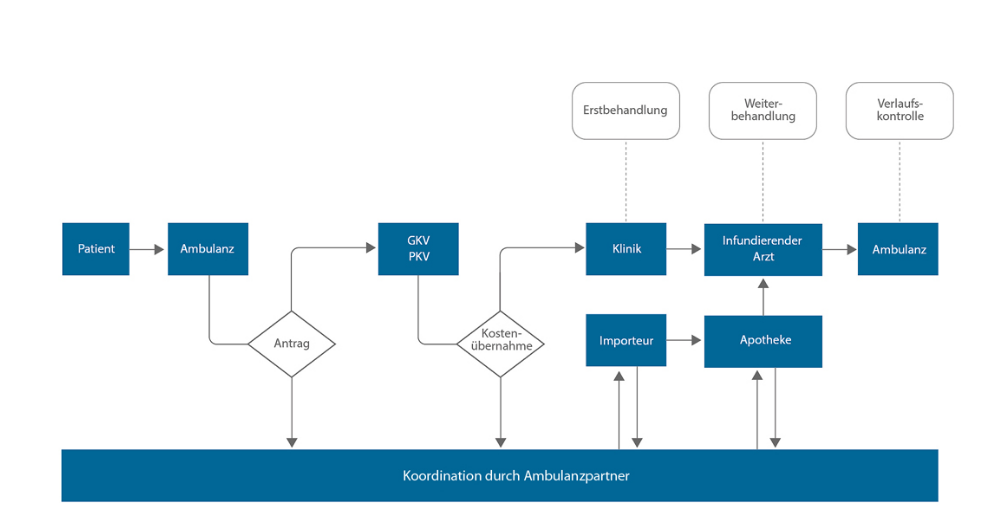
Abbildung 2: Organisatorische Unterstützung der Edaravone-
Behandlung durch Ambulanzpartner. GKV, PKV: gesetzliche, private Krankenversicherung
Monitoring des Krankheitsverlaufs und der Behandlungszufriedenheit
Die therapeutische Wirkung von Edaravone wurde in klinischen Studien anhand der ALS-Schweregrad-Skala (ALSFRSr) ermittelt. Somit ist die ALSFRSr-Skala relevant für die Festlegung individueller Therapieentscheidungen.
Im EMP werden die ALSFRSr-Skala und andere Selbstbewertungsdaten strukturiert erfasst. Patienten werden regelmäßig eingeladen, telefonisch oder online den ALSFRSr zu erheben. Aus dem ALSFRSr-Verlauf ergeben sich wichtige Informationen über den Behandlungs- und Versorgungsbedarf des Patienten. Weiterhin werden die pseudonymisierten ALSFRSr-Werte akademischen Forschungseinrichtungen und forschenden Unternehmen zur Verfügung gestellt. Dabei sollen offene Forschungsfragen zu Edaravone beantwortet werden.
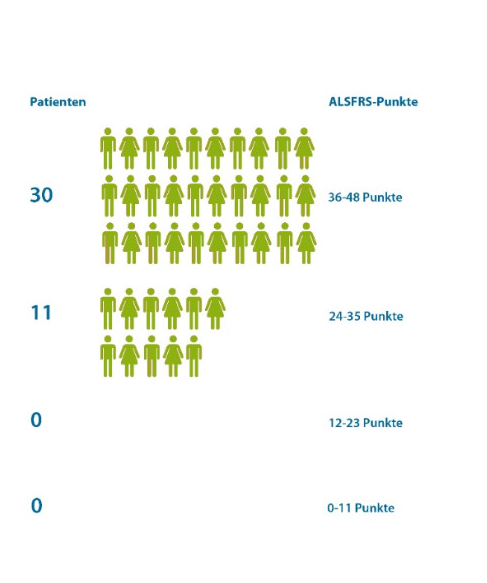
Abbildung 3: Betroffenheit bei Behandlungsbeginn anhand der ALSFRS-Skala. Der durchschnittliche Erkrankungsstatus von Patienten im Ambulanzpartner-Netzwerk liegt bei Beginn der Behandlung mit Edaravone bei 38 ALSFRSr-Punkten (min. 24, max. 46 Punkte). Quelle: Ambulanzpartner Registerstudie, Stand 01.09.2018
Patientenbewertung von Edaravone
Frage:
Sie nehmen Edaravone ein. Wir möchten Sie bitten, uns mitzuteilen, wie wahrscheinlich es ist, dass Sie Edaravone einem Freund (m/w) oder Kollegen (m/w) weiterempfehlen würden.
Auf einer Skala von 0 bis 10 Punkten:
10 = Eine Empfehlung ist höchst wahrscheinlich
0 = Eine Empfehlung ist absolut unwahrscheinlich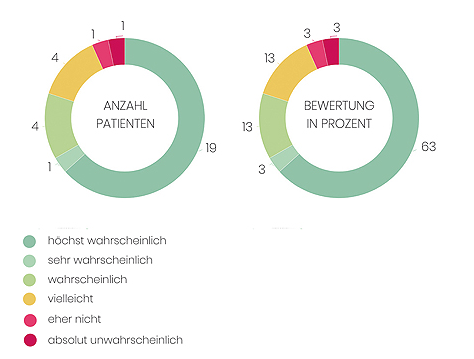
Abbildung 4: Patientenbewertung von Edaravone. Gesamtteilnehmer: 30 Patienten mit ALS.
Quelle: Ambulanzpartner Registerstudie, Stand 01.09.2018.
Wie erfolgt die Teilnahme am EMP?
Wenn Sie am Edaravone-Management-Programm teilnehmen möchten, können Sie uns telefonisch (030-81031410) oder per Email (koordination@ambulanzpartner.de) kontaktieren.
Ihr Ambulanzpartner-Team



