Edaravone is approved in Japan under the name Radicut and in the USA under the trade name Radicava for the treatment of amyotrophic lateral sclerosis (ALS). Despite the lack of approval for edaravone in Europe, the drug is also available in Germany as a single import (in accordance with Section 73 of the German Medicines Act).
Studies with edaravone show a moderate effect on the course of ALS. A challenge in the treatment with edaravone is that this drug is administered intravenously and requires regular infusions.
With the aim of answering open questions about the care of edaravone in ALS, the leading ALS centers in Germany are participating in a registry study on user experience and patient satisfaction with edaravone. In the period from July 2017 to January 2018, 30 ALS patients from the outpatient partner network were recruited to take part in an online survey on edaravone. The NPS patient satisfaction scale was used for this purpose.
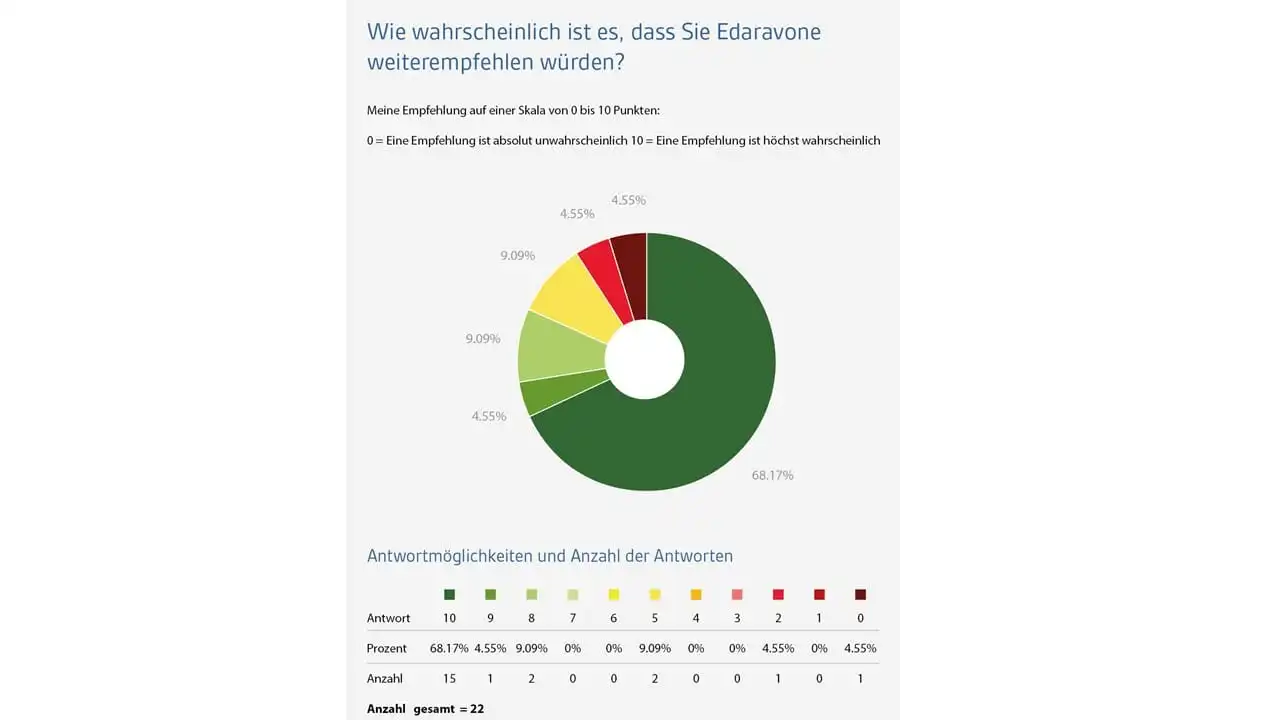
The Net Promotor Score(NPS) measures the likelihood of recommending edaravone to others by asking the following question: “How likely is it that you would recommend edaravone to a friend or colleague who has ALS?” The answers are given on a nominal scale between 0 (absolutely unlikely to recommend) and 10 (highly likely to recommend) scale points. An initial interim evaluation shows – despite the complex circumstances of intravenous drug administration – a high likelihood of recommending edaravone. Over 72% of patients stated that they were likely to recommend edaravone (10 or 9 points).
The outpatient partner survey also included results on treatment satisfaction (TSQM-9) and disease progression (ALS Functional Scale, ALS-FRS) were collected. The data on the ALS-FRS and TSQM-9 will be presented for the first time at a scientific meeting at the annual conference of the German Society for Clinical Neurophysiology and Functional Imaging (DGKN) on March 16, 2018 in Berlin.
This website uses cookies to improve your experience. We'll assume you're ok with this, but you can opt-out if you wish. Read More