This website uses cookies to improve your experience. We'll assume you're ok with this, but you can opt-out if you wish. Read More
Tag: ALS-Functional rating scale

Patient survey on THC:CBD in people with ALS
The THC:CBD patient survey is a systematic survey of 130 patients with amyotrophic lateral sclerosis (ALS), in which important findings on experience and satisfaction with treatment with cannabis-containing medicines containing tetrahydrocannabinol (THC) and cannabidiol (CBD) are obtained. Cannabis-containing treatment for ALS Medicines containing cannabis are used for severe illnesses when conventional medicines are not sufficiently…
Patient survey on THC:CBD in people with ALS
The THC:CBD patient survey is a systematic survey of 130 patients with amyotrophic lateral sclerosis (ALS), in which important findings on experience and satisfaction with treatment with cannabis-containing medicines containing tetrahydrocannabinol (THC) and cannabidiol (CBD) are obtained. Cannabis-containing treatment for ALS Medicines containing cannabis are used for severe illnesses when conventional medicines are not sufficiently…

Patients make intensive use of the online ALSFRS questionnaire – 1,000 scores already recorded
The 1,000 ALSFRS questionnaire was submitted online via Ambulanzpartner.de last weekend. We are delighted that the online self-assessment of the course of the disease is being used so intensively by patients. The 1,000 ALSFRS score was collected by Bernd König. “Your work is extremely important to me, especially in this situation,” writes Bernd König in…

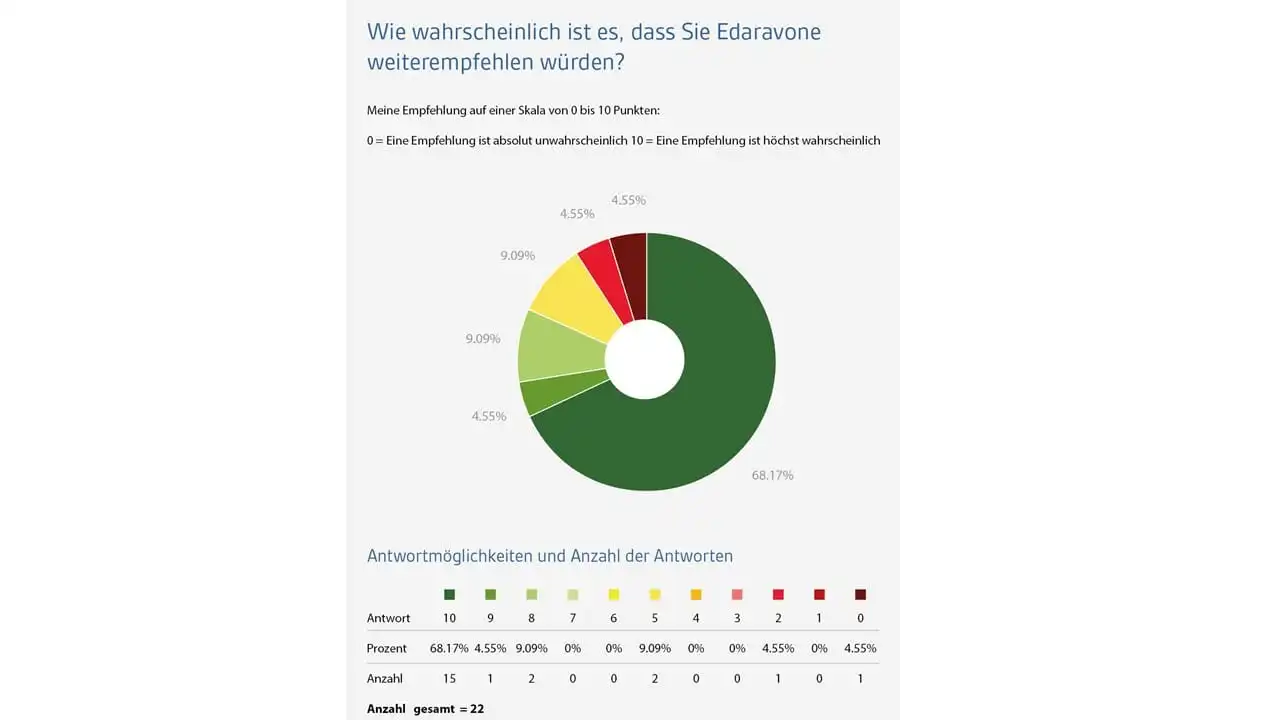
High satisfaction with edaravone (Radicut) in patients with amyotrophic lateral sclerosis (ALS)
Edaravone is approved in Japan under the name Radicut and in the USA under the trade name Radicava for the treatment of amyotrophic lateral sclerosis (ALS). Despite the lack of approval for edaravone in Europe, the drug is also available in Germany as a single import (in accordance with Section 73 of the German Medicines…