The Id-ALS study is a research program to identify genetic alterations in people with ALS. The study is organized by outpatient partners. The Alfried Krupp Hospital in Essen as well as the ALS outpatient clinic of the Charité - Universitätsmedizin Berlin have already started the genetic screening program of the Id-ALS study in December 2021. The integration of further 15 ALS centers is planned in the course of the next 6 months. This will involve the genetic testing of 1,000 patients by the end of 2022. Patients under care at any of the registered ALS study centers will be eligible to participate in this study.
The goal of the study is to identify genetic alterations in people with ALS who do not have a family history of ALS ("sporadic ALS"). Affected individuals with a positive family history may also participate in the study. This study is based on past research findings that genetic alterations in the genes SOD1, C9orf72, FUS and TARDBP are present in about 10% of all people with ALS. Identification of genetic alterations is becoming increasingly important as new drugs are in development that aim to therapeutically target SOD1, C9orf72, and FUS.
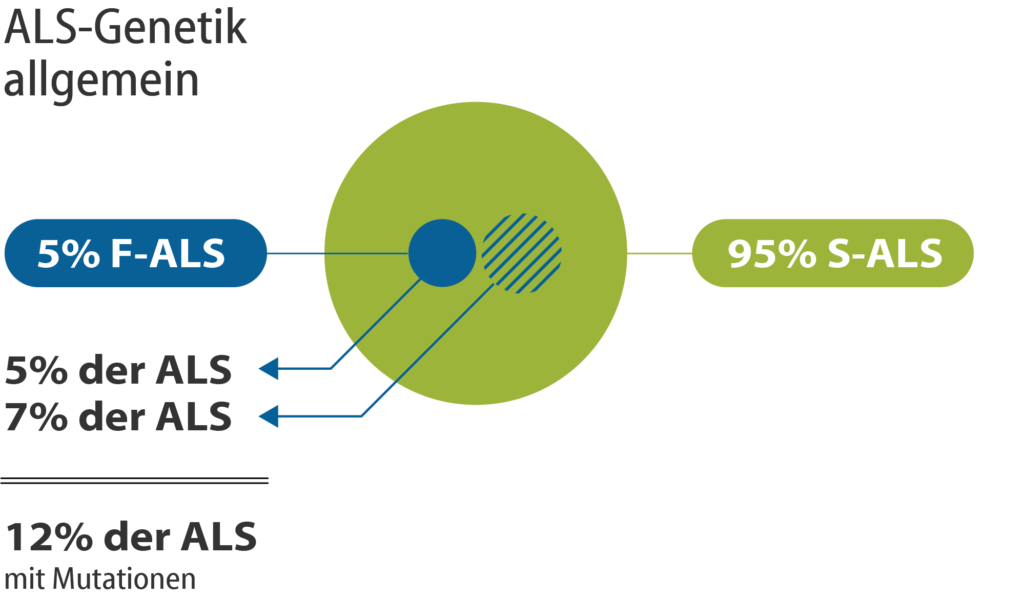
Figure 1: In the vast majority of affected persons (95%) with sporadic ALS (S-ALS), there is no information about a previous ALS disease in another family member. About 5% of all ALS patients can be assumed to have familial ALS (F-ALS), i.e. there is inheritance within a family. Although S-ALS patients do not have a family history of ALS, genetic changes can also occur in about 7% of S-ALS patients. These alterations are mostly identified in the course of research projects. With targeted genetic diagnostics, gene mutations are detectable in about 12% of people with ALS.
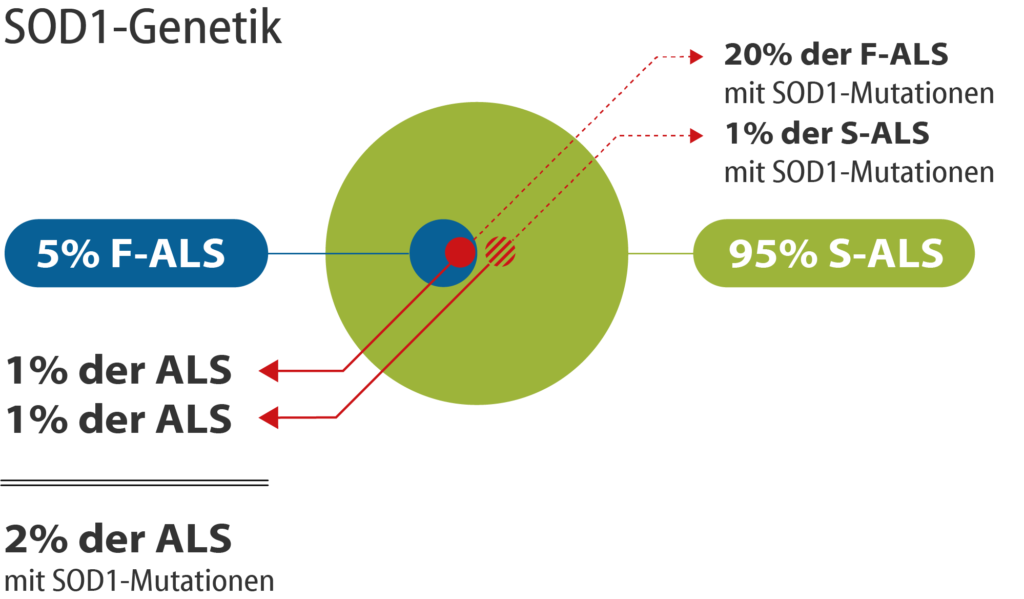
Figure 2: Mutations in the superoxide dismutase (SOD1) gene are known for about 20% of familial ALS patients (F-ALS). About 1% of the non-hereditary sporadic forms of ALS (S-ALS) carry a mutation in the SOD1 gene. Thus, a mutation in the SOD1 gene is present for 2% of all people with ALS.
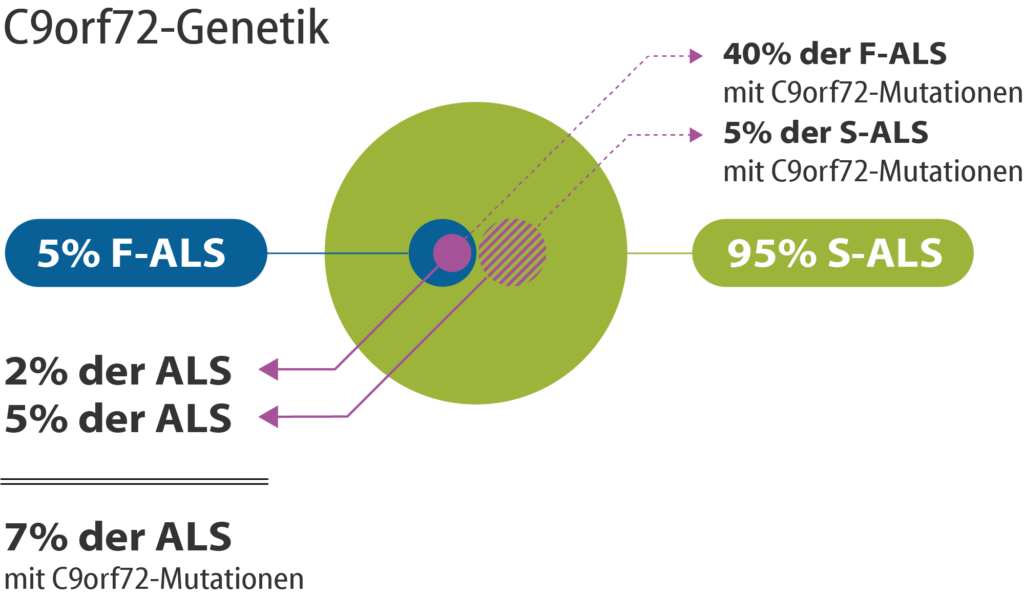
Figure 3: Genetic alterations in the C9orf72 gene are known for about 40% of familial ALS patients (F-ALS). About 5% of the non-hereditary sporadic forms of ALS (S-ALS) carry a mutation in the C9orf72 gene. Thus, a mutation in the C9orf72 gene is present for 7% of all people with ALS.
The study is one of the most extensive genetic studies in Germany and on an international scale. The study will be conducted in the ALS-Podcast #13 (Genetische Testung und Id-ALS Studie) presented. The importance of genetic factors in ALS and their relevance for future therapies will be discussed in the episodes of the ALS-Podcasts #4 (Genetics of ALS) , #8 (ALS-Gene) sowie #13 (Id-ALS Studie) discussed in more detail. Further information on the purpose of the study, the procedure, the time involved, the possibilities of reporting the results, and the planned study centers are described below.
Why does the Id-ALS study exist?
The purpose of this study is to explore the following relationships:
What is the process of the study?
A detailed description of the study procedure can be found in the document "Study Information for Patients", which is handed out to each participant. After information and education about the study, genetic counseling is performed, which is highly variable in terms of time and is essentially determined by the family constellation and the need for information of the study participants. The study consists of data collection and the collection of a blood sample. Besides disease-related data, the exact family history (possible family members with ALS, dementia or other neuropsychiatric diseases) will be collected. Furthermore, the ALS Functional Scale (ALSFRS-R) and a short questionnaire on the expectation towards a future genetic ALS therapy are collected. This is followed by a blood sample from a vein, which is sent to a human genetics laboratory for genetic analysis. The result will be communicated at a later date. The consultation, questioning and blood sampling are carried out by the study physician or by a study assistant ("study nurse").
What is the time commitment associated with study participation?
The study consists of data collection and the collection of a blood sample. For the genetic analysis, a blood sample (10 ml) is required from the vein, which is taken by medical professionals. The time required for the interview and blood sampling is between 20 and 30 minutes. In addition, there is the genetic counseling, which is very variable in time and is essentially determined by the family constellation and the need for information of the study participants. In addition, there is the survey of the ALS Functional Scale (ALSFRS-R) from home, which is repeated at intervals of 3 months. The survey on the ALSFRS-R scale takes 5-10 minutes.
At what interval is the genetic analysis performed?
Genetic analysis of the 4 selected genes (SOD1, C9orf72, FUS and TARDBP) is performed only once. The study results, combined with genetic counseling, are communicated with a time delay - usually several months later, after receipt of the analysis results from the genetic laboratory in Vienna. The ALS Functional Scale Survey (ALSFRS-R) is repeated at 3-month intervals over a 12-month period. The survey of the ALS Functional Scale (ALSFRS-R) is provided by self-assessment from home, via the personal account on the Ambulance Partner platform or via the mobile smartphone app, "ALS App".
How long will the study be conducted?
The project is designed for 2 years and has been supported by the ethics committee of the Charité with a positive vote until December 2023. Should research questions still be open after three years, an extension of the study would be requested.
How is the genetic analysis performed?
For the genetic analysis, a blood sample is taken from a vein (usually from the arm vein) at the study center. The blood sample is given a pseudonym at the study center and sent together with the questionnaires (which also only contain a pseudonym; without recognizable personal data) to Ambulanzpartner in Berlin. There, the completeness of the data is checked and centrally sent to the genetic laboratory of ARCHIMED Life Science GmbH ("ARCHIMED Life") in Vienna, Austria. In a first step the analysis genetic material is extracted from the blood sample. In a second step, the actual genetic analysis is performed using special molecular biological methods. Genetic alterations in the following genes are examined: SOD1, C9orf72, FUS and TARDBP. The genetic laboratory ARCHIMED Life is quality controlled and certified for research work as well as for the performance of human genetic diagnostics.
How is the result of the genetic analysis communicated?
The genetic laboratory provides the pseudonymized result to the study coordination of Ambulanzpartner via study software. Prior to consent, study participants were asked to decide between different options when communicating the genetic study results:
This determination is documented in the study consent form and in the study software. Depending on this specification, the pseudonymized genetic result is forwarded to the study center via the study software. There, the pseudonym is decoded and the genetic result - in the context of genetic counseling - is communicated to the patients.
Where will the Id-ALS study be conducted
The Id-ALS- study is offered at several ALS outpatient clinics in Germany. The following ALS outpatient clinics are participating in the study (named in alphabetical order)
Why is the use of the "ALS app" provided during the study?
The ALS Functional Rating Scale revised (ALS-FRS-R), a questionnaire on 12 motor functions that may be affected in ALS, systematically assesses the severity of the disease and the progression of ALS. The ALS Functional Scale (ALSFRS-R) survey is provided by self-assessment from home, through the personal account on the Ambulance Partner platform, or through the mobile smartphone app, "ALS App". The ALSFRS-R survey is repeated at 3-month intervals over a 12-month period. The survey takes 5-10 minutes to complete. The regular collection of the ALSFRS-R scale is intended to establish a scientific link between disease progression and genetic status. This will address the question of whether the course of ALS, as measured by the ALSFRS-R scale, shows differences between patients depending on genetic findings.
How do I get more information about the Id-ALS study?
Written study information, which is handed out and discussed at the study center, provides information about the procedures of the study. In addition, clarification of open questions by a study physician and study coordinator - also at the respective study center - is possible and necessary. Study participation requires informed consent, genetic counseling and prior clarification of open questions.
Thank you for your interest. If you have any questions about the study, please do not hesitate to contact Ms. Peggy Schumann.

p.schumann@ambulanzpartner.de
Telefon 030-81031410
